Abstract
Background: Standard chemoimmunotherapy for first-line treatment of follicular lymphoma (FL) achieves high rates of disease control but is not curative and carries significant toxicities including prolonged immunosuppression that may attenuate response to vaccinations (Marcus et al., NEJM 2017). While proteasome inhibitors have shown modest activity in R/R FL (Goy et al., JCO 2005), limited data address their use frontline. The comparatively favorable toxicity profile and convenient oral dosing of ixazomib support its investigation in this space.
Methods: We evaluated ixazomib and its combination with short-course rituximab (R) for FL as part of an open-label, phase II investigator-initiated trial at the University of Washington / Fred Hutch Cancer Research Center / Seattle Cancer Care Alliance (NCT 02339922). Eligibility included an indication for treatment per NCCN guidelines and no prior standard systemic FL therapy. Ixazomib was administered at 4 mg orally once a week until disease progression or unmanageable toxicity. One course of R at 4 weekly doses of 375 mg/m 2 was added during the 7 th 28-day cycle, after an initial 6-cycle "window" on ixazomib alone. Available pretreatment formalin-fixed, paraffin-embedded tissue biopsies were subjected to RNA extraction by standard methods and gene expression profiling (GEP) using the NanoString™ PanCancer IO 360 panel to query pathways in proteasomal degradation and lymphomagenesis. Standard GEP quality control and data processing were performed with the ROSALIND® platform. Patients vaccinated per standard of care for COVID-19 while actively receiving ixazomib and ≥ 6 mo after completing R were evaluated for serologic response ≥ 2 weeks after the final dose of vaccine using the Roche Elecsys® Anti-SARS-CoV-2 S assay against the spike protein receptor binding domain.
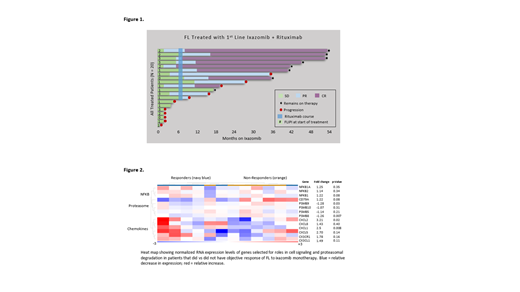
Results: Twenty pts began therapy between Feb 2017 and January 2020. All had grade I/II FL and FLIPI score was 2 in 20% and ≥ 3 in 20%; FLIPI score in all other patients was 0 or 1. Eleven (55%) pts met GELF criteria for high tumor burden disease including 6 (30%) pts with a tumor mass ≥ 7 cm. Median follow-up was 32.1 months (range 5.7 - 51.6). The ORR by Lugano criteria was 35% (CR 5%) during the ixazomib window and 65% (CR 45%) overall. At data cut (June 15, 2021) all patients were alive and 8 (40%) remained progression-free on treatment (Figure 1). By KM estimate, median PFS was 25.8 mo and median DOR was not reached at a median follow-up of 29.6 mo. As expected, high-grade treatment-related AEs were infrequent for ixazomib and R, including grade ≥ 3 events in 3 unique pts (15%; diarrhea, transaminitis, and cytopenias). No grade ≥ 4 or serious AEs were observed. Toxicities led to study-directed drug interruptions in 4 (20%) pts and dose reduction to ixazomib 3 mg weekly in 2 pts (10%). Higher ORR to ixazomib monotherapy was associated with FLIPI > 1 (p = 0.04) and, by exploratory GEP, downregulation of components of proteasomal degradation and upregulation of NF-KB and chemokine signaling (Figure 2). High tumor burden by GELF (p = 0.89) and tumor mass ≥ 7 cm (p = 0.26) were not associated with ORR to ixazomib. All 6 of 6 patients evaluated to date for response to COVID-19 vaccination, administered at a median of 32.5 mo (range 7.0 - 41.0) after last dose of R, achieved positive anti-spike protein antibodies (median anti-S 163.8 AU/mL, range 13.3 - 1139); none was diagnosed with COVID-19.
Conclusions: The simple outpatient regimen of weekly oral ixazomib and the addition of 4 doses of R shows significant long-term activity with low toxicity in untreated FL. Extended DOR is achievable especially in patients who respond to ixazomib monotherapy. Ixazomib efficacy was associated with higher FLIPI scores and gene expression signatures implicated in proteasomal degradation and B-cell signaling pathways. Ixazomib deserves further investigation as a biomarker-driven therapeutic in untreated FL, particularly as an option that prioritizes outpatient management and serologic responsiveness to immunization.
Graf: MorphoSys: Consultancy, Research Funding; TG Therapeutics: Research Funding; AstraZeneca: Research Funding; BeiGene: Research Funding; GSK: Research Funding. Lynch: Incyte: Research Funding; SeaGen: Research Funding; TG Therapeutics: Research Funding; Cyteir: Research Funding; Genentech: Research Funding; Morphosys: Consultancy; Bayer: Research Funding; Juno Therapetics: Research Funding. Ujjani: Gilead: Honoraria; ACDT: Honoraria; Kite, a Gilead Company: Honoraria; Adaptive Biotechnologies: Research Funding; Janssen: Consultancy; TG Therapeutics: Honoraria; Loxo: Research Funding; Atara Bio: Consultancy; Epizyme: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Cowan: Bristol Myers Squibb: Research Funding; Secura Bio: Consultancy; Sanofi Aventis: Consultancy, Research Funding; Harpoon: Research Funding; Abbvie: Consultancy, Research Funding; Nektar: Research Funding; GSK: Consultancy; Cellectar: Consultancy; Janssen: Consultancy, Research Funding. Smith: Millenium/Takeda: Consultancy; ADC Therapeutics: Consultancy; KITE pharm: Consultancy; Incyte Corporation: Research Funding; Beigene: Consultancy, Research Funding; Merck Sharp & Dohme Corp: Research Funding; Portola Pharmaceuticals: Research Funding; Ignyta (spouse): Research Funding; Genentech: Research Funding; AstraZeneca: Consultancy, Research Funding; Karyopharm: Consultancy; De Novo Biopharma: Research Funding; Bristol Myers Squibb (spouse): Research Funding; Incyte: Consultancy; Acerta Pharma BV: Research Funding; Bayer: Research Funding; Ayala (spouse): Research Funding. Shadman: Mustang Bio, Celgene, Bristol Myers Squibb, Pharmacyclics, Gilead, Genentech, Abbvie, TG Therapeutics, Beigene, AstraZeneca, Sunesis, Atara Biotherapeutics, GenMab: Research Funding; Abbvie, Genentech, AstraZeneca, Sound Biologics, Pharmacyclics, Beigene, Bristol Myers Squibb, Morphosys, TG Therapeutics, Innate Pharma, Kite Pharma, Adaptive Biotechnologies, Epizyme, Eli Lilly, Adaptimmune , Mustang Bio and Atara Biotherapeutics: Consultancy. Libby: Janssen: Consultancy, Research Funding; BMS: Research Funding; Genentech: Research Funding; GSK: Research Funding. Godwin: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company; Pfizer: Research Funding. Gopal: Genetech: Consultancy, Honoraria, Research Funding; Karyopharm: Consultancy, Honoraria; Incyte: Honoraria; Acrotech: Consultancy, Honoraria; ADC Therapeutics: Consultancy, Honoraria; Kite: Consultancy, Honoraria; MorphoSys: Honoraria; Epizyme: Consultancy, Honoraria; Gilead: Consultancy, Honoraria, Research Funding; Teva: Research Funding; IGM Biosciences: Research Funding; Bristol Meyers Squibb: Research Funding; Astra-Zeneca: Research Funding; Cellectar: Consultancy, Honoraria; Agios: Research Funding; Takeda: Research Funding; Merck: Consultancy, Honoraria, Research Funding; Servier: Consultancy, Honoraria; I-Mab bio: Consultancy, Honoraria, Research Funding; Nurix Inc: Consultancy, Honoraria; Beigene: Consultancy, Honoraria; SeaGen: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding.
Ixazomib is not approved for use in follicular lymphoma.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal